Coverage and analysis of leaked Heartland Institute documents published by DeSmogBlog has, for perfectly understandable reasons, focussed squarely on what their contents appear to tell about the organisation’s active involvement in the promotion of climate change denialism, netting the story the inevitable #deniergate hashtag on Twitter.
I say appear to tell us as the Heartland Institute has now issued a statement on its website in which it claims that the ‘smoking gun’ document, a confidential memo entitled ‘2012 Heartland Climate Strategy’ is a fake, even if the strictly factual information in the memo appears to be entirely consistent with the ‘Proposed Budget’ document that was leaked at the same time. This statement also suggests that another of the documents may have been altered, although it hasn’t as yet identified which of the documents its referring to and, somewhat predictably, also includes some fairly vague threats of legal action to go with a request that ‘activists, bloggers and other journalists should “immediately remove all of these documents and any quotations taken from them, especially the fake “climate strategy” memo and any quotations from the same, from their blogs, Web sites, and publications, and to publish retractions.”
There’s a biblical story about seeds falling on stoney ground that immediately comes to mind but, be that as it may, the statement does confirm exactly how these documents got into the wild; via an employee who mistakenly emailed them to an anonymous third party who’d manage to the blag them into thinking the request has come from a member of the organisation’s board; thus proving yet again that the one truly sustainable and inexhaustible resource this planet has is human stupidity.
The climate change-related elements of this story will run their natural course and as both the media and most of the main climate science bloggers seems to be fully on top of the story there seems little need for me to add my own comments.
However, a quick mooch through the budget document did turn up a bit of information that did rather pique my interest, a line item which shows that $644,000 has been allocated to a new project under the name ‘Free to Choose Medicine’ – or it could be $677,127, if you look at the projected expenses table, and it may even be $751,255 if you look at the projected departmental budget (which includes overheads). Whatever – the one settled figure throughout is that the organisation is looking to raise a cool $1,000,000 in the next twelve months in order to bankroll the project, half of which has already been pledged by Bartley J Madden, the ‘brains’ behind the entire project.
But what is it?
Well, if you’ve been drinking the Heartland Institute’s Kool-Aid then it’s a proposal for market-based dual track system of drug development and testing that will deliver ‘better drugs, sooner, at lower cost’.
If you look at it in a rather more sceptical fashion then its… well, let me walk you through Madden’s proposed system and you’ll see.
Before we get in the detail, I should give a little additional background on the main players in this project.
It is, as I’ve already mentioned, the brainchild of Bradley J Madden, a Heartland Policy Advisor who bills himself, on his extremely brief LinkedIn profile as an ‘Independent Philanthropy Professional.
Madden has a degree in mechanical engineering and an MBA from UC Berkeley which he obtained on either side of a spell in the army in which he served in Vietnam. His main line of business up until his retirement in 2003 was in the financial services sector where he specialised in the development of corporate valuation models, although the last position he held before retirement was that of managing director of Credit Suisse.
Free to Choose Medicine’s current project director is Vincent Galbiati whose LinkedIn profile clearly hasn’t been updated for quite a while as it still bills him as being the President and CEO of the Northwest Indiana Forum, a position he left back in January 2010. As with Madden, Galbiati’s background is in financial services and, interesting, includes a five year period as raw material trader at Koch Industries. His time at the Northwest Indiana Forum was not without its own controversial elements, as the article reporting on his decision to step down from the role notes:
Galbiati’s tenure at the Forum was marked by a broadening of its mission and activities. He also guided it through one of its biggest public controversies, when it used both public and private money for a mass media campaign in support of expanding the South Shore commuter railroad.
Critics contended the $130,000 in public money the Forum received from the Northwest Indiana Regional Development Authority should not have been used to promote a project that had not yet been approved by taxpayers.
Predictably, the same article goes on to add:
The Forum accounted for use of the public money but would not reveal the identity of private donors.
You do surprise me.
Background checking the principal characters also threw up one unintentionally funny moment as a note in the budget document indicates that Galbiati wasn’t the Institute’s first choice for the role of project director.
Horace Cooper, who was a legislative specialist working out of our Washington DC office since 2010, was let go mid-year after we concluded he wasn’t the right guy to lead a new and expanded Free to Choose Medicine project. He will be replaced in 2012 by Vince Galbiati. Horace remains an unpaid senior fellow.
Not the right guy, eh – I wonder why?
WASHINGTON — A former official with the Labor Department turned conservative commentator pleaded guilty Wednesday in a corrupt lobbyist case, saying he wanted to put the whole thing behind him.
Horace Cooper, also a one-time aide to former Republican Rep. Dick Armey when he was majority leader of the House, pleaded guilty to falsifying a document when he did not report receiving gifts from lobbyists Jack Abramoff and Neil Volz in 2003.
When Cooper was chief of staff for the Employment Standards Administration, he was required by law to report any such gifts. An indictment alleges he took expensive meals, concert tickets and sports tickets.
Masters of understatement then.
Cooper was eventually sentenced to 3 years probation, 300 hours community service and a $500 fine and also managed to plead poverty with enough success to persuade the trial judge to waive interest on the fine.
So that’s the background, but what about this dual track system that Madden has apparently developed – what’s that all about?
To understand Madden’s proposals we need to run quickly through clinical trials 1o1.
All new drugs are put though a four phase trial process as follows.
In Phase 1 the drug is given to young healthy volunteers – although they’re actually paid an ‘inconvenience fee’ to serve as lab rats – as a first stage test of safety, tolerability and ‘dose-ranging’, i.e. trial subjects are given escalating dosages during the trial from which researchers can work out the best and safest dose and the point at which the drug become to poisonous to administer.
Phase 2 are conducted on small groups of patients who have the condition or illness that the drug is intended to treat and at this stage researchers continue the safety tests started at phase one and also assess how well the drug works. Phase 2 trials tend to conducted either as case series studies or, in some case, as randomised controlled trials.
Provided that the drug successfully passes phases 1 and 2 it can then move on to a phase 3 trial, which is a much larger randomised controlled trial in patients with the condition/illness that the drug is intended to treat and its this phase that it intended to provide the definitive assessment of the drug’s effectiveness relative to the current best available treatment.
Pass all three phases successfully – and at phase 3 this usually means two successful trials providing solid evidence that the drug is either more effecting that existing treatments or, at least, as effective but with fewer/a lower risk of side effects, and your drug will get the approval it needs for i to reach the market.
Phase 4 is usually referred to as post-marketing surveillance, under which the safety of the drug is monitored once it reaches the marketplace in order to pick up any problems that may not have been identified during the earlier trial phases.
This is a long and brutally expensive process with drugs often (but not always) taking anything from 12-18 years to reach the marketplace and so, at least in theory, a system that could safely short-circuit this process and bring effective drugs to market in a shorter period of time and at a lower cost could indeed deliver better treatments, sooner and at a lower cost to the patient/taxpayer, depending on where you are in the world and how the local healthcare system is funded.
However there’s a catch or two to navigate before we get to ‘better, sooner, cheaper’.
Madden’s plan would not alter the core trial process. New drugs would still be required to make their way through the full three-phase trial system in order to obtain and FDA marketing licence. However, the system would be modifed to introduce a new ‘free to choose’ track at the mid-point of phase II trials at which point onto which pharmaceutical companies could place new drugs if they appeared to have performing well at this point and this would allow what the Institute describes as ‘less risk-averse’ patients to gain access these drugs, in consultation with their doctor, while the main trial process continued in the background.
This would certainly mean that some patients would gain access to these new drug much sooner than would be the case were they to wait for them to receive full FDA approval, but does that necessarily mean that they’ll be getting access to better treatments?
A recent (2011) study of clinical trial success rates by the Biotechnonology Industry Organisation and BioMedTracker, which covers 4275 drugs and 7,300 indication at any trial phase during the period from October 2003 to 2010 gives us some extremely useful contextual information on success rates at different trial phases.
On the whole, the pharmaceutical industry seems to be pretty good at getting new drugs through phases 1 and 3 of the trial process. The success rate at phase 1 is a healthy 63%, while at phase 3, where trials are limited only to those drugs that make it through phases 1 and 2, the current success rate is around 80%, although only around 55% of new drugs make the grade at the first time of asking and without the FDA asking the developer to go back and carry out an additional trial or two or provider some additional data before they’ll award the drug a full licence.
At phase 2, however, things look rather different. The success rate at phase 2 is only 33% – 2 out of every 3 drugs that enter phase 2 trials fail to make the grade and the majority of drop-outs occur after the midpoint of the phase trial, the point at which, under Madden’s dual track plan, new drugs could be placed onto the ‘Free to Choose’ track.
Unfortunately, the data I have, which comes from a presentation and not the full study, doesn’t provide much information on the reasons why drugs were failing to make the grade at phase 2 beyond a single graph which shows that success rates for secondary indications were, at 23%, lower at phase 2 than they were primary indications (41%), a pattern that is found at all trial phases. All we can say is that its likely that some of the failures are due to safety issues, i.e. side effects, some because drug prove to be ineffective or, at least, less effective than drug which are already available and some for other reasons. Its not that uncommon for trials to show that a new drug is effective and safe but only within a sub-group of the patients with the condition/illness its intended to treat and this may act to limit the potential market for the drug to a size at which further development just isn’t financially viable as there would little or no prospect of the developer recouping their costs of the phase 3 trials required to obtain a marketing licence.
Crucially, what we also don’t know is how many of the drugs that do fail at phase 2 actually fail at or before the midpoint of the trial, information which is going to be of critical importance to these ‘less risk-averse’ patients, and their doctors, when deciding whether to access drugs places onto Madden’s ‘Free to Choose’ track, this being the most basic information necessary to assess the risk of a new drug turning out to be a dud.
So, the main part of the deal for patients is that they may be able to get much quicker access to new drugs of uncertainty safety and efficacy – peachy – but what about the pharmaceutical industry and, perhaps, doctors as well, What has Madden got to offer them?
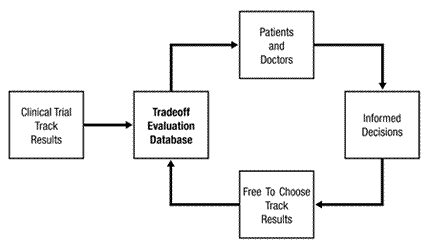
Well, for starters, a database called TED, or what Madden calls a ‘Tradeoff Evaluation Database – and here’s the start of the Institute’s pitch for TED from their own website:
For us to be able to judge if the benefit from a new drug exceeds the benefit from an approved drug and is worth the risk – i.e., for us to be informed well enough to be able to evaluate the tradeoff – we and our doctors would need relevant, up-to-date information. Under my proposal, that information would be accessible on the Internet through a Tradeoff Evaluation Database (TED), as shown in Figure 2.
Say hi to TED…
So TED would pull together data from clinical trials, which at phase 2 could mean either case series data or data from an RCT, and data from patients on the ‘Free to Choose’ track which, at best, would amount to more uncontrolled case series data and this would supposedly enable doctor and patient to make ‘informed decisions’ about the efficacy of these new drugs, all of which goes to show that Madden’s knowledge and understands of the clinical trial process is somewhere on a par with that of Dr Jack Schitt.
Disclaimer – The ‘Dr Jack Schitt’ referred to above is a satirical figure of speech and does not refer to any real physician, living or dead.
At best, this system could improve the chances of spotting unwanted side effects and other safety issues somewhat earlier in the trial process, which is great for the developer as it may save them expense of finding out the same information at a later, and much more expensive, stage of the clinical trial process but, obviously, that’s not such a hot outcome for the patients who get to find out about these problems the hard way.
In terms of evaluating the efficacy of new drugs against existing treatments, however, the system is a complete dud.
If you look at most standardised evidence grading system you’ll quickly that case series data is typically ranked 2-3 grades lower than evidence from a properly designed randomised controlled trial, the kind of trial that has been conducted at phase 3 in order for new drugs to obtain FDA approval. Indeed, the data from the ‘Free to Choose’ track may not even make the grade as acceptable case series data given that it comes from what amounts to a wholly uncontrolled, self-selecting group of patients. At best, the system may provide nothing of value in terms of assessing the efficacy of new drugs, at worst it could prove to introduce all manner of uncontrolled confounding factors in the trial process and generally create an unholy mess of the evidence base, making genuinely informed treatment decisions next to impossible.
And that’s not all, as TED is intended to capture rather more than just patient outcomes.
Legislation to implement Dual Tracking should specify that participation in the Free To Choose Track requires not only that doctors input treatment results to TED, but also that patients permit doctors to transmit the patient’s genetic and biomarker information to TED. Over time, this would create a treasure trove of public data that would greatly benefit pharmaceutical research.
TED might be next to useless as a clinical tool but its hell of way to grab people’s personal data for the pharmaceutical industry’s benefit and, best off all, the patients would even be paying for the privilege of handing over their genetic and biomarker information to Big Pharma and, I’m sure, anyone else who can come up with a plausible sounding excuse for gaining access to TED’s data warehouse, function creep being what it is.
Come to think of it, who said anything about function creep…
At no charge, a government-operated TED would provide the information needed to make informed decisions about what is in patients’ best interests. Private-sector companies (e.g., Google, Microsoft, IBM, and such) would have a profit incentive to sell customized “consumer reports” that would further help patients and doctors. Consumer reports could pinpoint subsets of patients who are most and least likely to benefit, forecast the probability of FDA approval, and provide head-to-head comparisons of Free to Choose drugs against relevant FDA-approved drugs.
So, the patient pays to hand over their personal data to the government who provides the data, free of charge, to the private sector so that they can sell back to doctors, and their patients, at a profit,
Sorry, did I not mention anything about paying for this system yet? Well here’s Madden’s plan…
Most private health insurance contracts cover only FDA-approved drugs. Lack of FDA approval could keep people with less financial means from obtaining the life-improving or life-saving drugs accessible via the Free To Choose Track. This is not a situation Americans would want, and we would pressure FDA to get more engaged with the needs of all patients.
One way to help FDA respond is for the Free To Choose Medicine Act to allow the agency to grant conditional approval for a new drug based on a combination of results from clinical trials and Free To Choose Track use. To maintain conditional approval, the developer would have to agree to complete Phase III trials and obtain conventional FDA approval within a reasonable time.
As I said, it’s the patients that end up paying for this whole shebang via their health insurance but at least the system will still require Big Pharma to put their drugs through the full trial process, won’t it?
TED would transfer knowledge (and power) to doctors and patients. This could disrupt enrollment in those clinical trials where knowledgeable doctors judge the drug to be tested as superior to approved treatments. The proper response from FDA should be to develop more innovative testing procedures that avoid unethical clinical trials and give top priority to today’s patient needs.
Before and during Phase III testing, TED would make public a vast amount of clinical trial and observational data. Some drugs would show strikingly effective treatment results compared to FDA-approved drugs. Then, many knowledgeable doctors may object to enrolling their patients in Phase III randomized control trials on the ethical grounds of not wanting to subject their patients to the risk of receiving an inferior drug or useless placebo.
You know, I’m beginning to wonder whether TED’s last name might not be ‘Bundy’.
Of course, without conducting properly designed phase III randomised controlled trials, we have no reliable way of knowing whether or not a new drug may actually be inferior to existing treatments or even just a placebo – and in the circumstances, placebos are not necessarily useless, but that’s a debate for another occasion.
The reasoning here is deliciously Orwellian – black really is white and vice versa.
You create a system under which patients can be given drug treatments that could be inferior to those already in the market and then get rid of the only means of finding out whether these treatments are inferior, RCTs, on the grounds that it would be unethical to subject patients to the risk of receiving inferior treatments.
Quick, give this man the Nobel Prize for bullshit before anyone catches on.
Now you might think that this all sounds just a bit risky given that Americans can be just a bit on the litigious side if, as could easily happen to some people, things don;t quite work out as well as they might have hope after exercising their freedom to choose, but fear not, the Heartland Institute have that one covered as well.
Drug developers would be unlikely to make new drugs in clinical testing available through a Free To Choose Track if they could be held liable for all side effects even if they were not negligent in developing, testing, or manufacturing. Consequently, as part of a Free To Choose Medicine Act, Congress would adopt legislation permitting patients to waive their right to sue drug developers under strict product liability as long as developers do not provide false or misleading information.
You knew that was coming, didn’t you.
So, to sum up the deal that the Heartland Insitute are offering for my American readers, it goes something like this…
You too can have access to better treatment, sooner and at a lower cost, just so long as you’re prepared to forget about having a healthcare system that actually operates as a healthcare system and are happy to sign up to one that operates as just a big old pharmaceutical laboratory…
…and guess who the rats are?
As you may have just noticed, this all comes in a package that the Heartland Institute are calling the ‘Freedom to Choose Medicine Act’ and its to promote this Act that they’re looking to pull in an addition $500,000 dollars, this year, to go with the cool half-a-million that Madden has already thrown into the kitty, which obviously prompts us to ask where, exactly, they expect to get this money from?
The answer can be found in another document from the package that was leaked to DeSmogBlog…
Free To Choose Medicine (FTCM) is a new project of The Heartland Institute. During 2011, Heartland’s president Joseph Bast wrote an 80-page proposal for the effort, and donor Bart Madden agreed to contribute half of the project’s $1 million/year budget. In January, a director for the project, Vince Galbriati, was hired.
We expect the FTCM to bring in at least $1 million in gifts from all donors, including Bart Madden. Approximately $500,000 in gifts will be from first-time donors. There are scores of businesses and trade associations with a keen interest in our effort, and many wealthy individuals have strong personal motivation to see faster access to potentially life-saving new drugs become a reality. Vince Galbriati is eager to “pitch” the proposal to investors in drug companies. Several current Heartland donors are already donating to the project, and will give more if they see progress being made.
Businesses, trade associations and many wealthy individuals with a ‘strong personal motivation’ to see shitloads of cash flying in the general direction of their offshore bank accounts, although you can rest assured that it’s really only the interests of the lab rats patients that they really take to heart.
In the final analysis, I think Madden’s proposal speaks for itself although I am just a little concerned about the name of the project. ‘Free to Choose Medicine’ doesn’t really trip lightly off the tongue. It’s lack that bit of ‘snap’ it needs to make it truly memorable.
Luckily enough, I have an idea that I think nicely sums up the character of the project to a tee.
Reading through the Heartland Institute’s shtick the one thing that really leapt out at me was just how closely the deal at the heart of the package, under which marks patients get to pay for unproven drug treatments of unknown safety and efficacy, resembles the business model that the Burzynski Clinic has been using for the last thirty years. In fact, a system which rooks patients into paying for new and unproven drug treatments while systematically seeking to undermine the FDA’s the clincial trial process at the same time can only be viewed as manna from heaven if you happen to be a thoroughly unprincipled but seriously entrepreneurial quack and, as such, a suitable and much snappier name for this project seems entirely obvious.
Ladies and gentlemen I give you ‘Burzynskicare™‘.
8 thoughts on “Heartland Institute to promote ‘Burzynskicare’ Project”